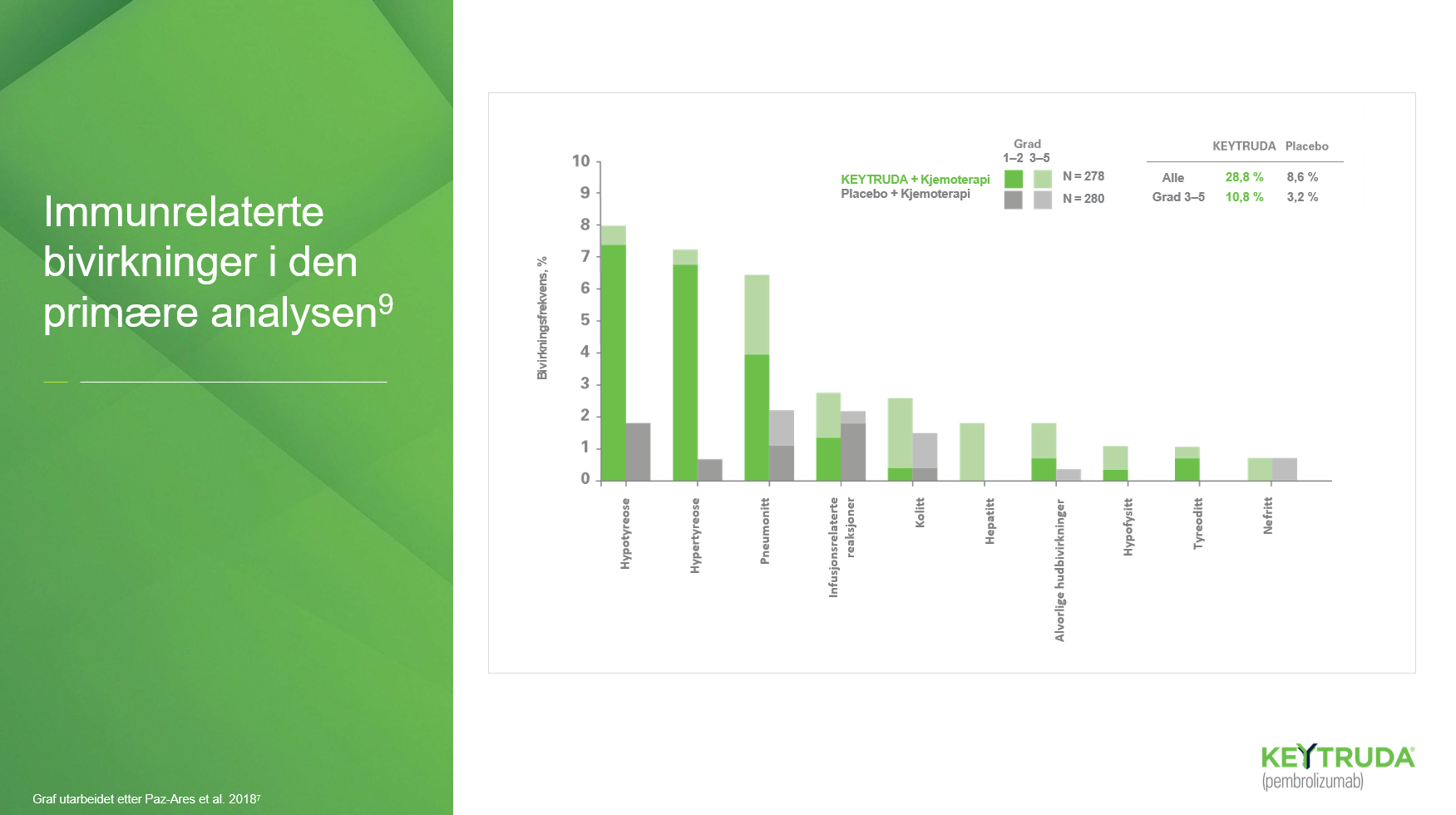
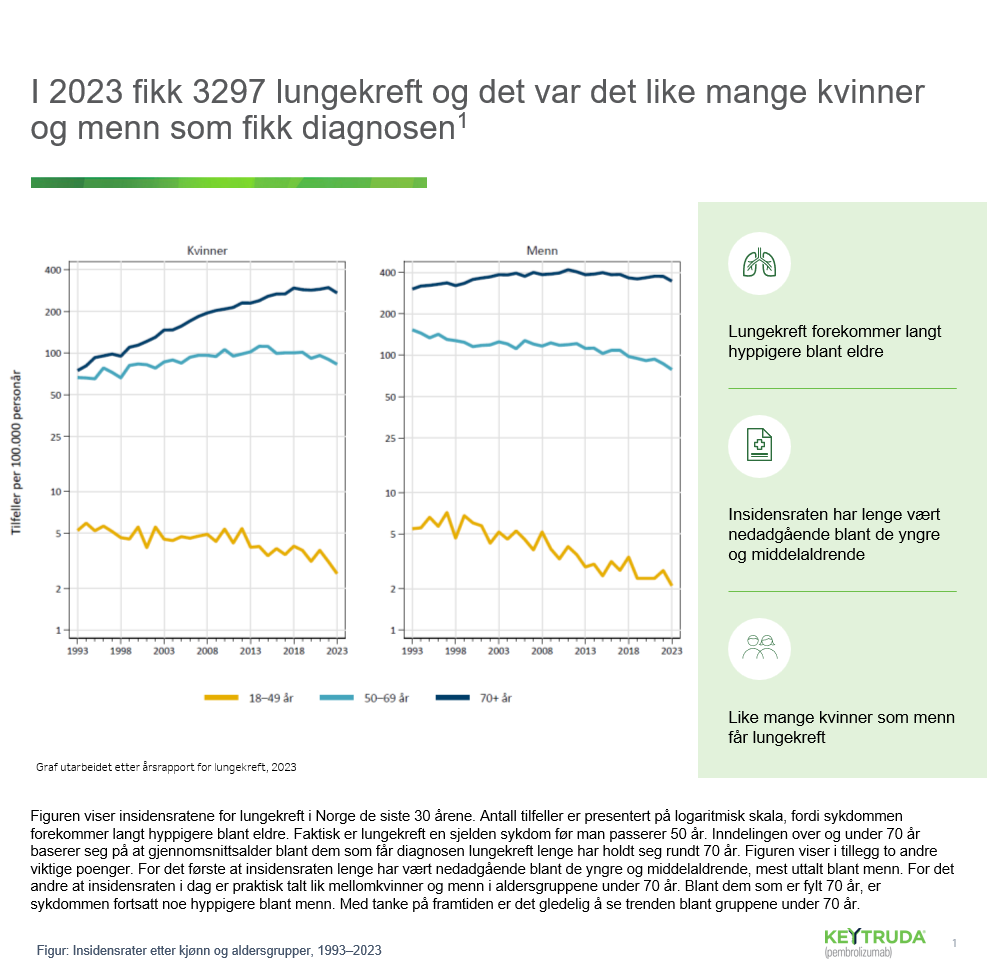
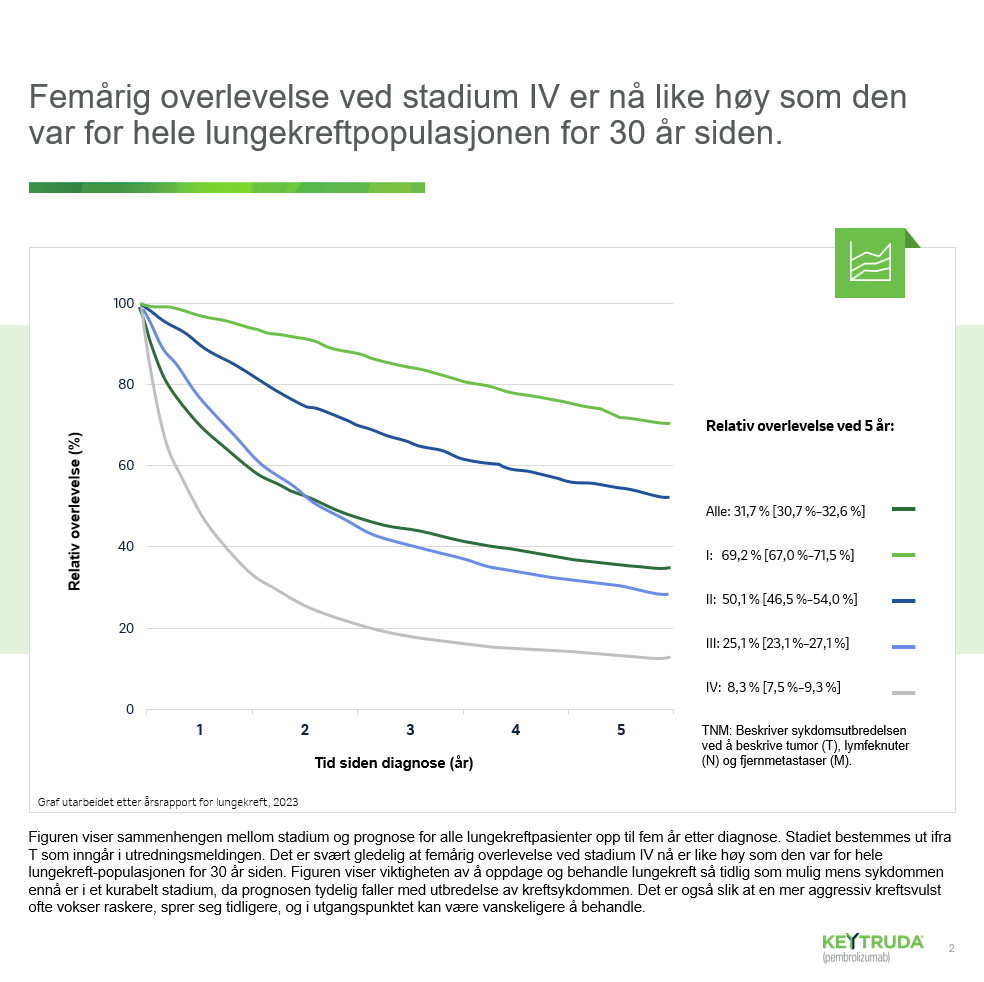
Lungekreft
Årsrapporten 2023

Se denne filmen om hva administrering hver 6.uke kan bety for dine pasienter og din avdeling
Se utfyllende informasjon i preparatomtalen, i utvalgt sikkerhetsinformasjon og bivirkninger.
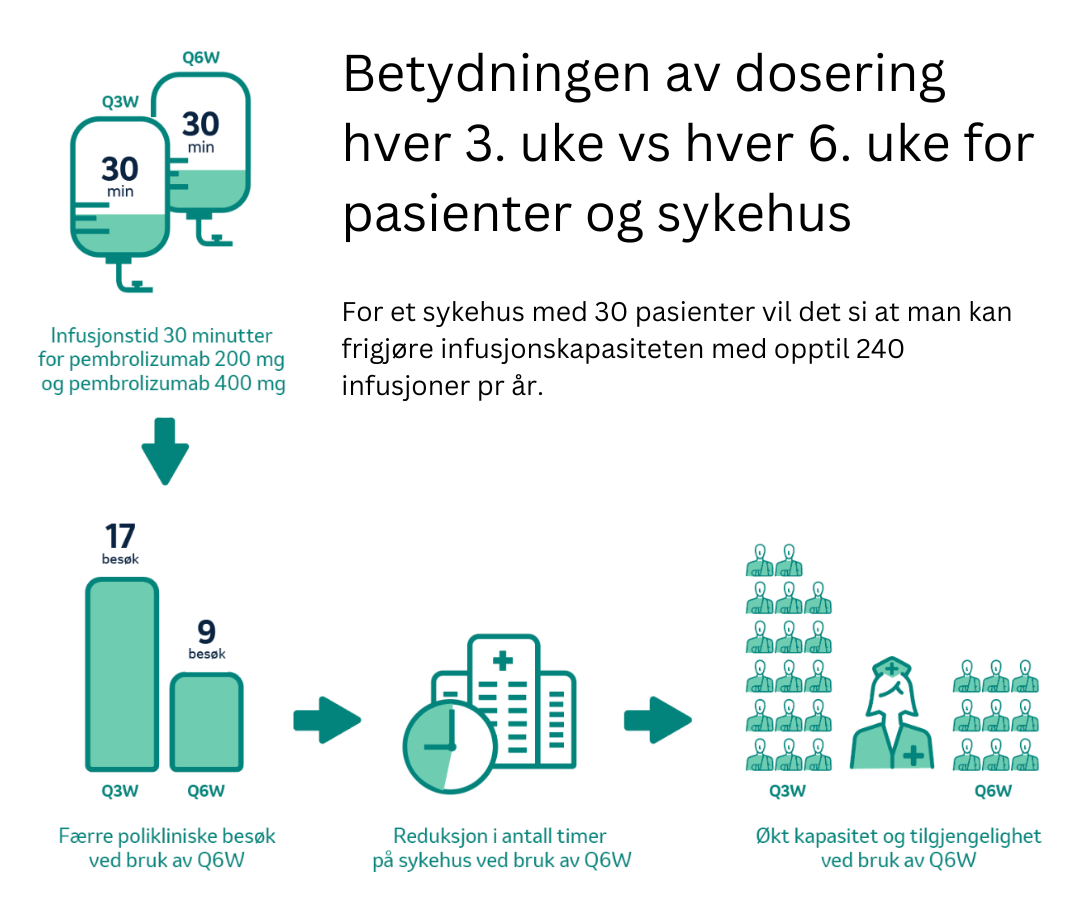
* Q3W = hver 3. uke, Q6W = hver 6. uke
Samarbeid pasientforening
Har du spørsmål rundt KEYTRUDA®?
Ta kontakt med oss.
022
Referanser
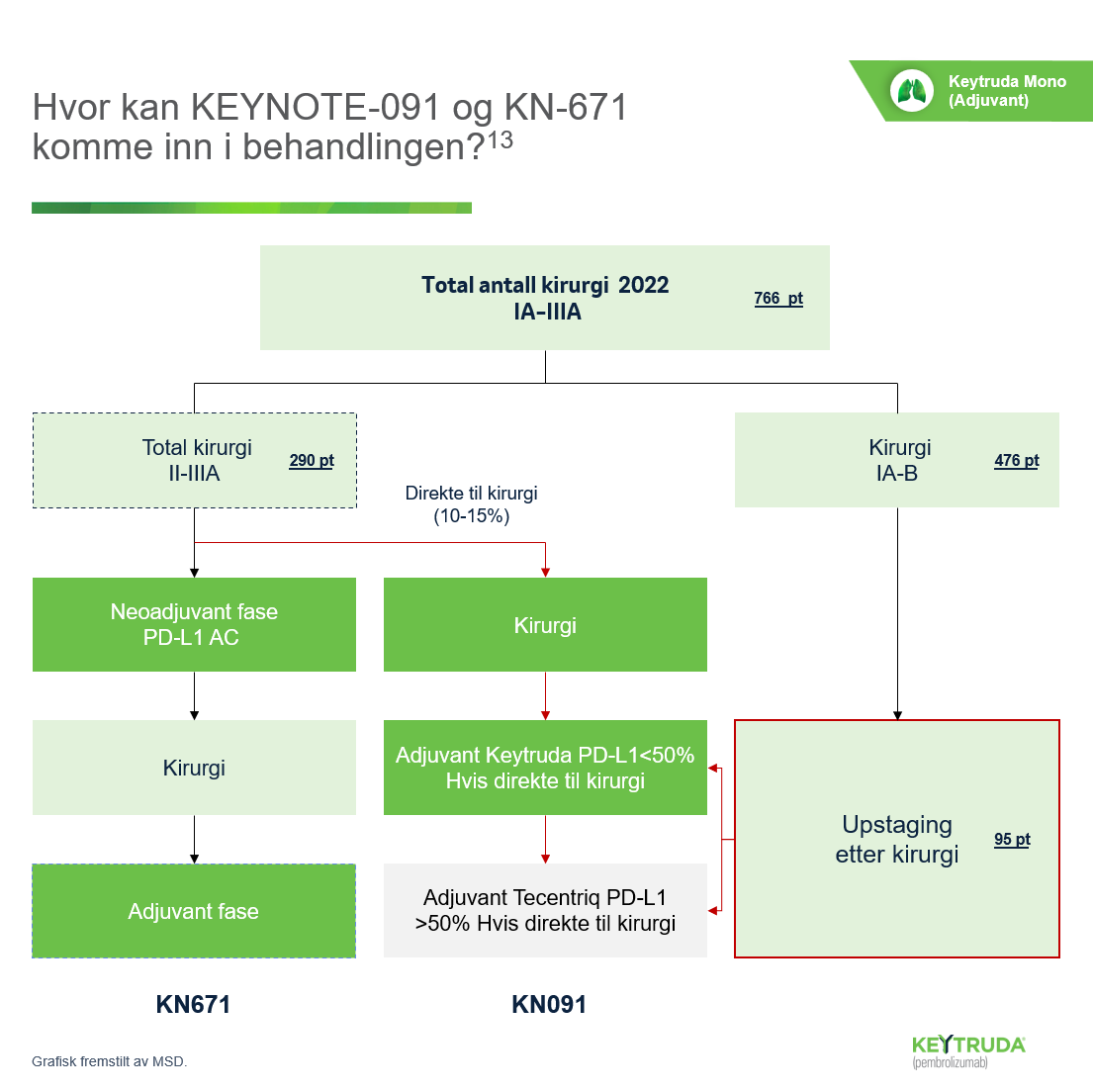
- O´Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARL/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022; 23; 19: 1274 – 86.
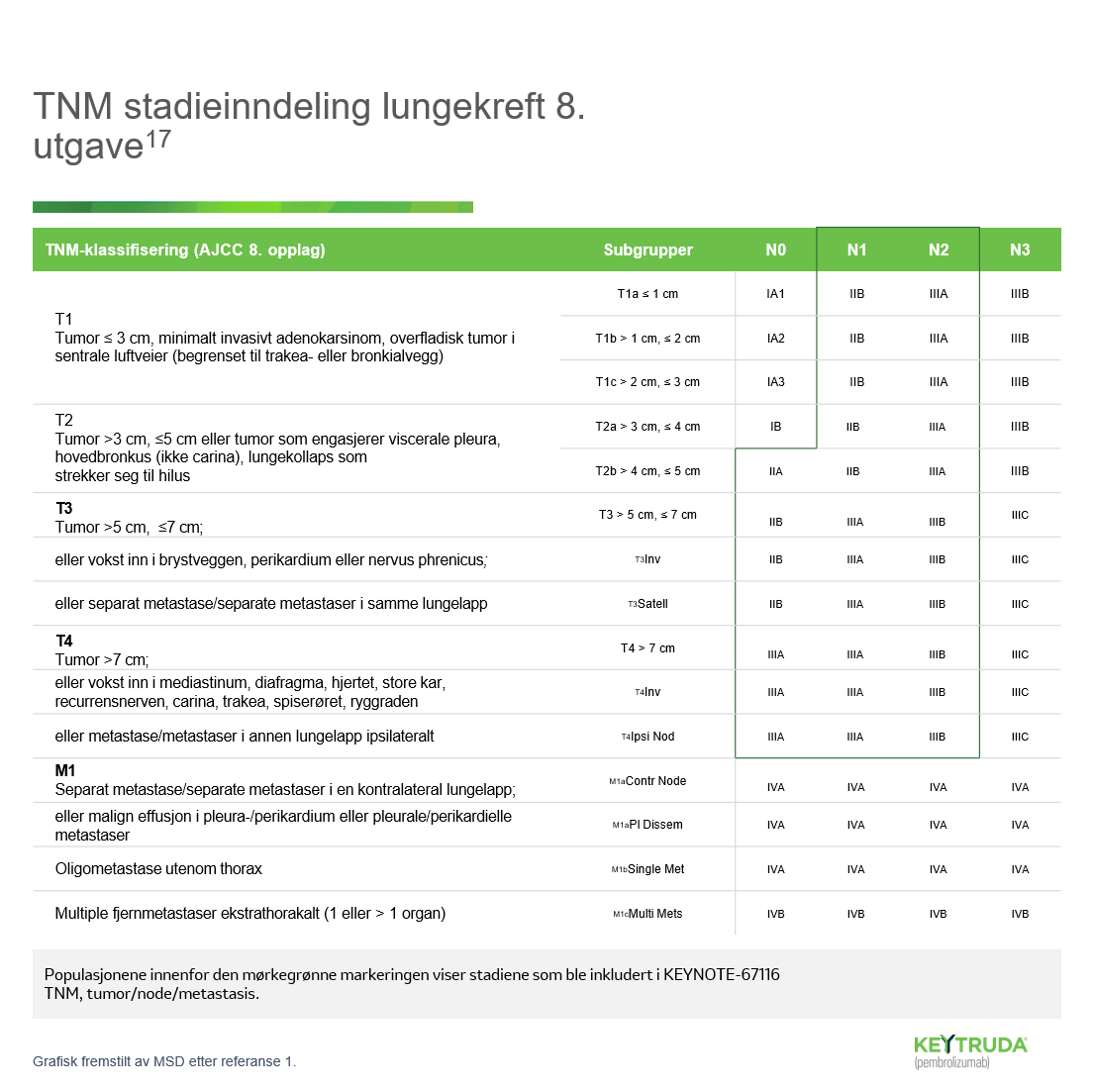
- Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S, et al.; KEYNOTE-671 Investigators. Perioperative Pembrolizumab for Early-Stage Non- Small-Cell Lung Cancer. N Engl J Med. 2023 Aug 10;389(6):491-503.
- Reck M et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375(19): 1823-1833.
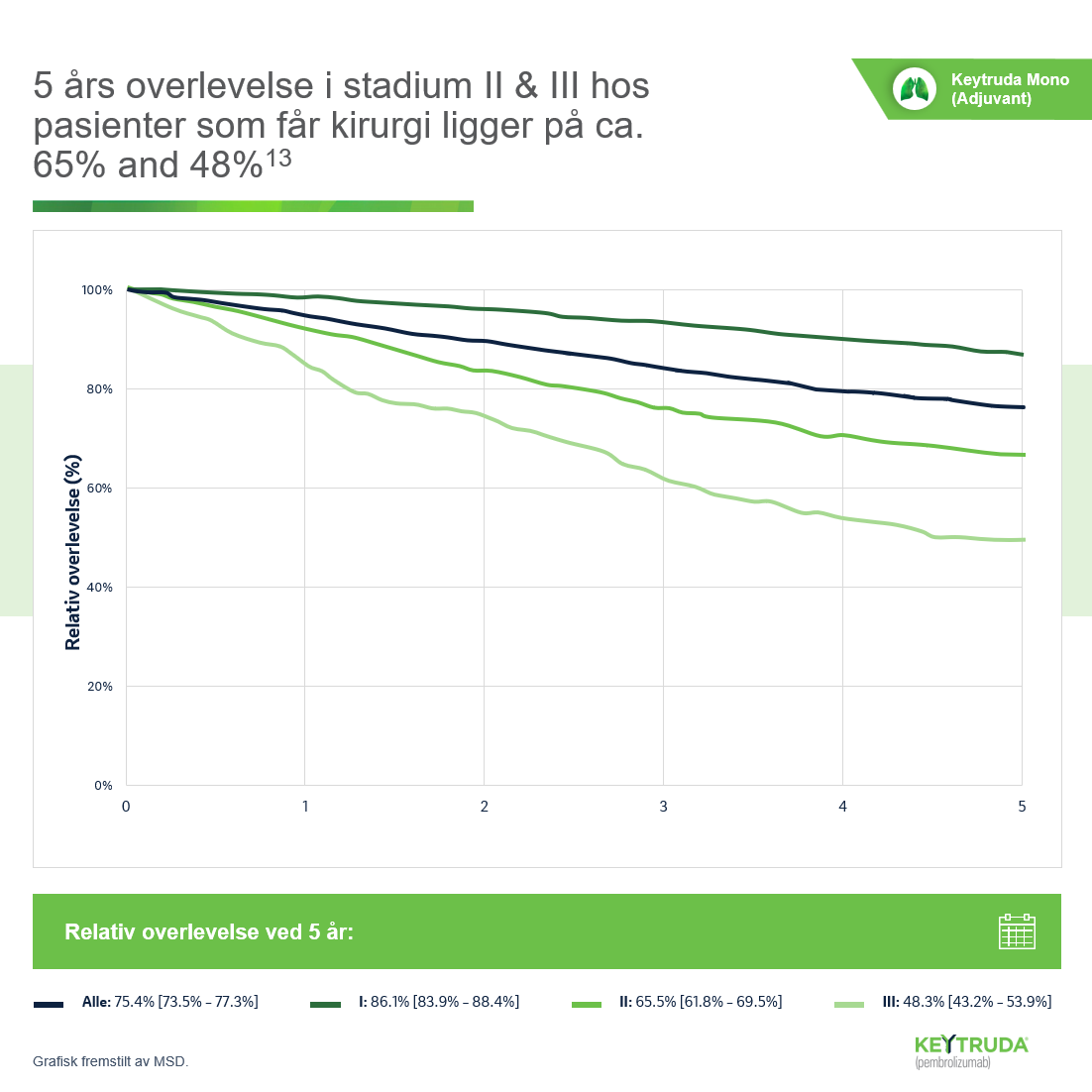
- Reck M et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small cell lung cancer with PD-L1 tumor proportion score ≥ 50 %. J Clin Oncol. 2021
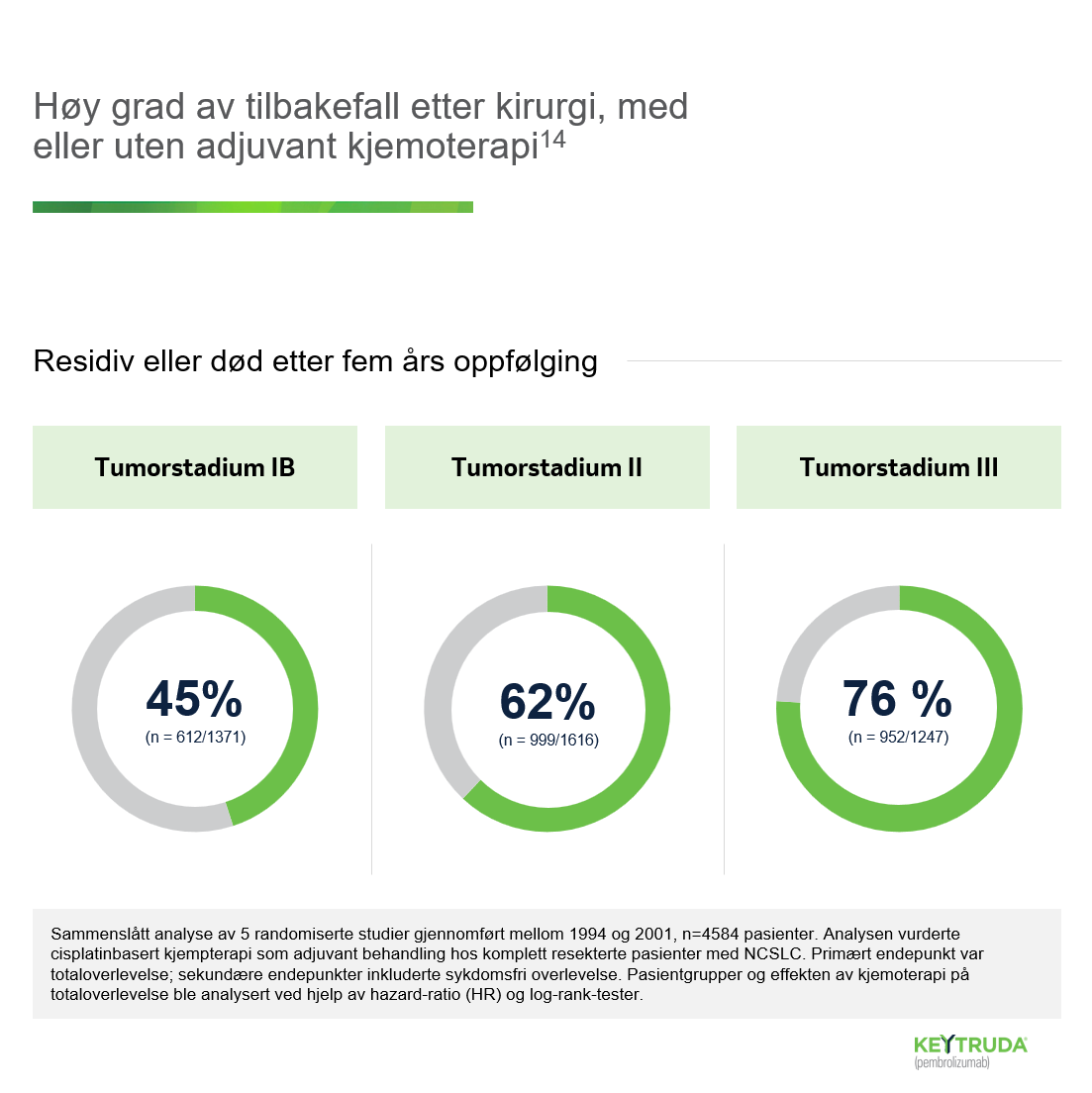
- Gandhi L et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018:378(22): 2078-2092
- M.C. Garassino et al. KEYNOTE-189 5 year update: First-line pembrolizumab + pemetrexed and platinum vs placebo + pemetrexed and platinum for metastatic nonsquamous NSCLC. Oral presentation ESMO 2022.
- Paz-Ares et al. Pembrolizumab plus chemotherapy for squamous non-small cell lung cancer. N Engl J Med. 2018;379(21): 2040-2051.
- Novello S, et al. 5-year update from KEYNOTE-407: Pembrolizumab plus chemotherapy in squamous non-small cell lung cancer (NSCLC). Oral presentation ESMO 2022.
- Årsrapport for lungekreft 2023, resultater og forbedringstiltak fra Nasjonalt kvalitetsregister for lungekreft. https://www.kreftregisteret.no/Registrene/Kvalitetsregistrene/Kvalitetsregister-for-lungekreft/ (publisert mai 2024, lest 15 august 2024)
- KEYTRUDA (pembrolizumab) SPC, August 2024 4.1, 4.2, 5.1