Karsinom i øsofagus
KEYTRUDA™ (pembrolizumab), in combination with platinum- and fluoropyrimidine-based chemotherapy, is indicated for the first-line treatment of patients with locally advanced unresectable or metastatic carcinoma of the oesophagus in adults whose tumors express PD-L1 with a CPS ≥10.1

Overall Survival:
- KEYTRUDA + cisplatin/5-FU reduced risk of death by 38% vs cisplatin/5-FU alone (HRa=0.62; 95% CI, 0.49–0.78; Pb<0.0001)2
- Median OSc: 13.5 months (95% CI, 11.1–15.6 months) with KEYTRUDA + cisplatin/5-FU vs 9.4 months (95% CI, 8.0–10.7 months) with cisplatin/5-FU alone2
- Events observed: 66.7% (124/186) with KEYTRUDA + cisplatin/5-FU vs 83.8% (165/197) with cisplatin/5-FU alone2
aBased on the stratified Cox proportional hazard model.
bOne-sided P value based on log-rank test stratified by geographic region (Asia versus Rest of the World) and tumor histology (Adenocarcinoma versus Squamous Cell Carcinoma) and ECOG performance status (0 versus 1).
cBased on Kaplan-Meier estimation.
5-FU = 5-fluorouracil; CI = confidence interval; CPS = combined positive score; ECOG = Eastern Cooperative Oncology Group; HR = hazard ratio; OS = overall survival; PD-L1 = programmed death ligand 1.
KEYTRUDA—an anti–PD-1 immunotherapy with over 25 indications approved since 20151

KEYTRUDA can be used with your choice of platinum-/fluoropyrimidine-based chemotherapy for advanced oesophageal (adenocarcinoma or squamous cell carcinoma) cancer
KEYTRUDA, in combination with platinum- and fluoropyrimidine-based chemotherapy, is indicated for the first-line treatment of patients with locally advanced unresectable or metastatic carcinoma of the oesophagus or HER2-negative gastroesophageal junction adenocarcinoma in adults whose tumors express PD-L1 with a CPS ≥10.1
In KEYNOTE-590, 51% of patients had tumors that expressed PD-L1 (CPS ≥10) based on the PD-L1 IHC 22C3 pharmDxTM Kit1
The efficacy of KEYTRUDA® was investigated in KEYNOTE-590, a multicenter, randomized, placebo-controlled trial that enrolled 749 patients with metastatic or locally advanced oesophageal or gastroesophageal junction (tumors with epicenter 1 to 5 centimeters above the GEJ) carcinoma who were not candidates for surgical resection or definitive chemoradiation. PD-L1 status was centrally determined in tumor specimens in all patients using the PD-L1 IHC 22C3 pharmDx kit. Patients with active autoimmune disease, a medical condition that required immunosuppression, or who received prior systemic therapy in the locally advanced or metastatic setting were ineligible. Randomization was stratified by tumor histology (squamous cell carcinoma vs adenocarcinoma), geographic region (Asia vs ex-Asia), and ECOG performance status (0 vs 1).
Patients were randomized (1:1) to one of the following treatment arms; all study medications were administered via IV infusion:
- KEYTRUDA® 200 mg on Day 1 of each 3-week cycle in combination with cisplatin 80 mg/m2 IV on Day 1 of each 3-week cycle for up to 6 cycles and FU 800 mg/m2 IV per day on Day 1 to Day 5 of each 3-week cycle, or per local standard for FU administration, for up to 24 months.
- Placebo on Day 1 of each 3-week cycle in combination with cisplatin 80 mg/m2 IV on Day 1 of each 3-week cycle for up to 6 cycles and FU 800 mg/m2 IV per day on Day 1 to Day 5 of each 3-week cycle, or per local standard for FU administration, for up to 24 months.
Treatment with KEYTRUDA® or chemotherapy continued until unacceptable toxicity or disease progression. Patients could be treated with KEYTRUDA® for up to 24 months in the absence of disease progression. The major efficacy outcome measures were OS and PFS as assessed by the investigator according to RECIST v1.1. The study prespecified analyses of OS and PFS based on squamous cell histology, CPS ≥10, and in all patients. Additional efficacy outcome measures were ORR and DOR, according to modified RECIST v1.1, as assessed by the investigator.
The study population characteristics were:
Among the 749 patients in KEYNOTE-590, 383 (51%) had tumours that expressed PD-L1 with a CPS ≥ 10 based on the PD-L1 IHC 22C3 pharmDxTM Kit. The baseline characteristics of these 383 patients were: median age of 63 years (range: 28 to 89), 41% age 65 or older; 82% male; 34% White and 56% Asian; 43% and 57% had an ECOG performance status of 0 and 1, respectively. Ninety-three percent had M1 disease. Seventy-five percent had a tumour histology of squamous cell carcinoma, and 25% had adenocarcinoma.
SAFETY OF KEYTRUDA + cisplatin/5-FU IN KEYNOTE-590
In patients with esophageal cancer, adverse events occurring in at least 20% of patients and at a higher incidence (≥2% difference) of Grades 3–5 severity for KEYTRUDA in combination with chemotherapy (cisplatin and 5-FU) compared to placebo and chemotherapy (cisplatin and 5-FU) were: vomiting (7% vs 5%), stomatitis (6% vs 3.8%), neutrophil count decreased (24.1% vs 17.3%), and white blood cell count decreased (9.2% vs 4.9%).2
Selected AE summary (ASaT)2:
Any AEs (ASaT): 370 (100%) patients with KEYTRUDA + cisplatin/5-FU vs 368 (99%) patients with cisplatin/5-FU alone
Led to discontinuation: 90 (24%) patients with KEYTRUDA + cisplatin/5-FU vs 74 (20%) patients with cisplatin/5-FU alone
Treatment-related AEs (ASaT): 364 (98%) patients with KEYTRUDA + cisplatin/5-FU vs 360 (97%) patients with cisplatin/5-FU alone
Grade ≥3: 266 (72%) patients with KEYTRUDA + cisplatin/5-FU vs 250 (68%) patients with cisplatin/5-FU alone
Led to death: 9 (2%) patients with KEYTRUDA + cisplatin/5-FU vs 5 (1%) patients with cisplatin/5-FU alone
Immune-mediated AEs and infusion reactions (ASaT): 95 (26%) patients with KEYTRUDA + cisplatin/5-FU vs 43 (12%) patients with cisplatin/5-FU alone
Grade ≥3: 26 (7%) patients with KEYTRUDA + cisplatin/5-FU vs 8 (2%) patients with cisplatin/5-FU alone
achemotherapy = cisplatin/5-FU
5-FU = 5-fluorouracil; AE = adverse event; ASaT = all subjects as treated.

In patients whose tumors expressed PD-L1 with a CPS ≥10
KEYTRUDA + cisplatin/5-FU achieved a statistically significant improvement in efficacy outcome measures of OS and PFS vs cisplatin/5-FU alone2
dBased on the stratified Cox proportional hazard model.
eOne-sided P value based on log-rank test stratified by geographic region (Asia versus Rest of the World) and tumor histology (Adenocarcinoma versus Squamous Cell Carcinoma) and ECOG performance status (0 versus 1).
fBased on Kaplan-Meier estimation.
gAssessed by investigator using RECIST v1.1.
hBased on the stratified Cox proportional hazard model.
iOne-sided P value based on log-rank test stratified by geographic region (Asia versus Rest of the World) and tumor histology (Adenocarcinoma versus Squamous Cell Carcinoma) and ECOG performance status (0 versus 1).
jBased on Kaplan-Meier estimation.
PFS = progression-free survival; RECIST v1.1 = Response Evaluation Criteria in Solid Tumors v1.1.
The study demonstrated a statistically significant improvement in PFS for all pre-specified study populations. In all patients randomized to KEYTRUDA in combination with chemotherapy, compared to chemotherapy the PFS HR was 0.65 (95% CI 0.55–0.76). The median follow-up time was 13.5 months (range: 0.5 to 32.7 months). A total of 32 patients aged ≥75 years for PD-L1 (CPS ≥10) were enrolled in KEYNOTE-590 (18 in the KEYTRUDA combination and 14 in the control). Data about efficacy of KEYTRUDA in combination with chemotherapy are too limited in this patient population.
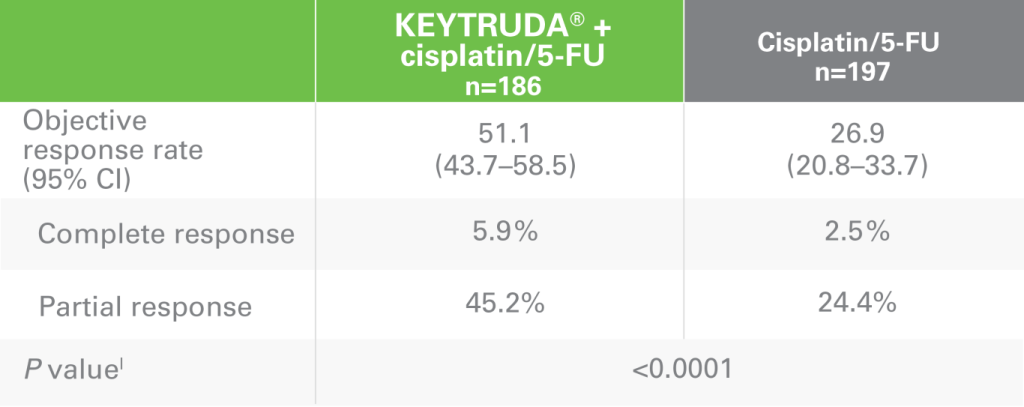
ORR with KEYTRUDA + cisplatin/5-FU vs cisplatin/5-FU alonek
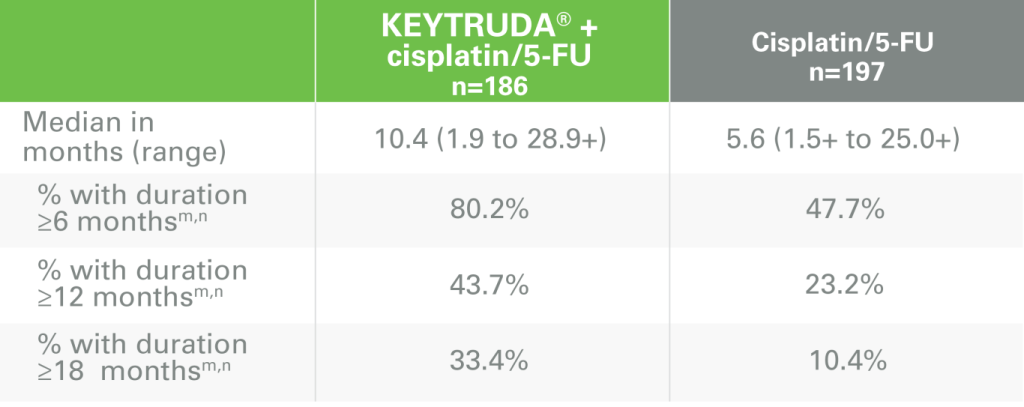
DOR with KEYTRUDA + cisplatin/5-FU vs cisplatin/5-FU alonek
kAssessed by investigator using RECIST v1.1.
lOne-sided P value for testing. H0: difference in % = 0 versus H1: difference in % >0.
mBest objective response as confirmed complete response or partial response.
nBased on Kaplan-Meier estimation.
ORR = objective response rate; DOR = duration of response; RECIST v1.1 = Response Evaluation Criteria in Solid Tumors v1.1.
In the first-line treatment of advanced oesophageal cancer with PD-L1 expression (CPS ≥10), KEYTRUDA + platinum-/fluoropyrimidine-based chemotherapy may be an option for your appropriate patients

Choose between two KEYTRUDA dosing options based on your choice of platinum-/fluoropyrimidine-based chemotherapy1
KEYTRUDA Dosing Options
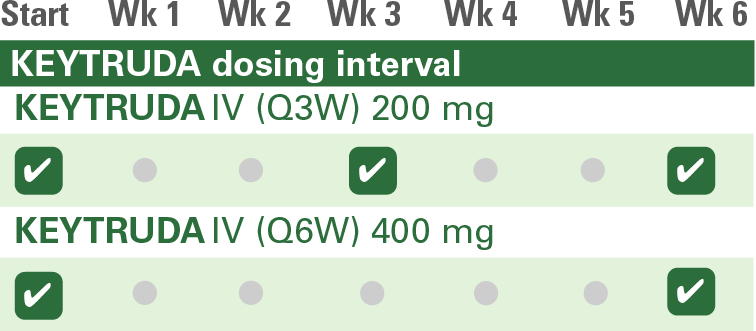
IV = intravenous; Q3W = every 3 weeks; Q6W = every 6 weeks.
Which dosing regimen of KEYTRUDA is appropriate for your patient with advanced oesophageal cancer?

A PD-L1 CPS ≥10 simplifies treatment decisions
The CPS scoring method evaluates the number of PD-L1–staining cells (tumor cells, lymphocytes, macrophages) relative to all viable tumor cells, multiplied by 1006
Patients With Certain Advanced Oesophageal Cancer
KEYTRUDA – platinum-/fluoropyrimidine-based chemotherapy is approved for patients with advanced oesophageal cancer whose tumors express PD-L1 with a CPS ≥101

Selected Safety Information
KEYTRUDA® (pembrolizumab) konsentrat til infusjonsvæske, oppløsning 25 mg/ml
Dosering og administrasjon: Behandling må initieres og overvåkes av lege med erfaring i kreftbehandling. Anbefalt dose av KEYTRUDA som hos voksne er enten 200 mg hver 3. uke eller 400 mg hver 6. uke administrert som intravenøs infusjon over 30 minutter. Pasienter skal behandles med KEYTRUDA inntil sykdomsprogresjon eller til uakseptabel toksisitet (og opptil maksimal behandlingsvarighet hvis spesifisert for indikasjonen). Ved administrering av KEYTRUDA som del av en kombinasjon med intravenøs kjemoterapi, bør KEYTRUDA administreres først. Ved bruk i kombinasjon, se preparatomtalen for samtidige behandlinger.
Pakning og Priser: Hetteglass 1×4 ml. Maksimal AUP: 41 553,80 NOK. Keytruda er inkludert i anbud for kreftlegemidler (LIS 2007) og selges med rabattert pris. Reseptgruppe: C, Refusjon: H-resept
Utvalgt sikkerhetsinformasjon:
Advarsler og forsiktighetsregler:
- KEYTRUDA skal ikke brukes under graviditet, hvis ikke den kliniske tilstanden til kvinnen gjør behandling med KEYTRUDA nødvendig.
- Fertile kvinner må bruke sikker prevensjon under behandling med KEYTRUDA og i minst 4 måneder etter siste dose med KEYTRUDA.
- Det er ukjent om KEYTRUDA skilles ut i morsmelk hos mennesker og en fordel- risikoevaluering før beslutning om ammingen skal opphøre eller om behandlingen med KEYTRUDA skal avsluttes/avstås fra må gjennomføres. Det er ingen kliniske data tilgjengelig for mulig effekt av KEYTRUDA på fertilitet.
Pasientkort: Alle forskrivere av KEYTRUDA må være kjent med informasjon til helsepersonell og retningslinjer for håndtering. Forskriver må diskutere risikoen ved behandling med KEYTRUDA med pasienten. Pasienten vil få et pasientkort ved hver forskrivning.
Bivirkninger: KEYTRUDA er vanligst assosiert med immunrelaterte bivirkninger. De fleste bivirkninger, inkludert alvorlige reaksjoner, reverserte etter initiering av hensiktsmessig medisinsk behandling eller seponering av pembrolizumab. Se Felleskatalogen for mer info om anbefalte behandlingsjusteringer ved immunrelaterte bivirkninger.
Andre svært vanlige (≥ 1/10) eller alvorlige bivirkninger i kombinasjon med kjemoterapi: nøytropeni, anemi, trombocytopeni, leukopeni, abdominalsmerter, diaré, kvalme, oppkast, forstoppelse, hypokalemi, nedsatt appetitt, insomni, perifer nevropati, hodepine, svimmelhet, dysgeusi, dyspné, hoste, artralgi, muskel og skjelettsmerter, myisitt, artralgi, fatigue, asteni, ødem, pyreksi, økt ALAT, økt ASAT, alopesi, kløe, utslett og alvorlige infusjonsrelaterte reaksjoner.
Interaksjoner: Bruk av systemiske kortikosteroider eller immunsuppressiva før oppstart av KEYTRUDA bør unngås på grunn av deres potensielle interferens med den farmakodynamiske aktiviteten og effekten til KEYTRUDA. Systemiske kortikosteroider eller andre immunsuppressiva kan imidlertid brukes etter oppstart av behandling med KEYTRUDA for å behandle immunrelaterte bivirkninger. Kortikosteroider kan også brukes som premedisinering, som profylaktisk antiemetika og/eller for å lindre kjemoterapirelaterte bivirkninger ved bruk av KEYTRUDA i kombinasjon med kjemoterapi.
Graviditet, amming, fertilitet: KEYTRUDA skal ikke brukes under graviditet, hvis ikke den kliniske tilstanden til kvinnen gjør behandling med KEYTRUDA nødvendig.Fertile kvinner må bruke sikker prevensjon under behandling med KEYTRUDA og i minst 4 måneder etter siste dose med KEYTRUDA.Det er ukjent om KEYTRUDA skilles ut i morsmelk hos mennesker og en fordel- risikoevaluering før beslutning om ammingen skal opphøre eller om behandlingen med KEYTRUDA skal avsluttes/avstås fra må gjennomføres. Det er ingen kliniske data tilgjengelig for mulig effekt av KEYTRUDA på fertilitet.
MSD (Norge) AS, PO box 1579 Vika, 0118 Oslo, tlf. 32 20 73 00.
Konsulter Keytruda SPC før forskrivning eller bruk for komplett informasjon om dosering, kontraindikasjoner, advarsler og forsiktighetsregler, interaksjoner og bivirkninger.
References: 1. Keytruda SmPC 2. Sun, J.-M., Shen, L., Shah, M. A., Enzinger, P., Adenis, A., Doi, T., Kojima, T., Metges, J.-P., Li, Z., Kim, S.-B., Cho, B. C., Mansoor, W., Li, S.-H., Sunpaweravong, P., Maqueda, M. A., Goekkurt, E., Hara, H., Antunes, L., Fountzilas, C., & Tsuji, A. (2021b). Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. The Lancet, 398(10302), 759–771. https://doi.org/10.1016/s0140-6736(21)01234-4 3. BMJ Best Practice Oesophageal Cancer 4. Marieb EN Hoehn K The digestive system in Human anatomy and Physiology 5. Schneider, P.M., Mönig, S.P. (2017). Siewert Classification of Adenocarcinoma of the Esophagogastric Junction: Still In or Already Out?. In: Giacopuzzi, S., Zanoni, A., de Manzoni, G. (eds) Adenocarcinoma of the Esophagogastric Junction. Springer, Cham. https://doi.org/10.1007/978-3-319-28776-8_7 6. Agient Technologies Inc PD-L1 IHC 22C3 pharmDx Interpretation Manual, Esophageal Canc

